Reaction That Has a Positive Δg Is Best Described as
3 It is endothermic and has a negative H. Phosphate the terminal phosphate -The γ-phosphate is the primary phosphate group on the ATP molecule that is hydrolyzed when energy is needed to drive anabolic reactions.
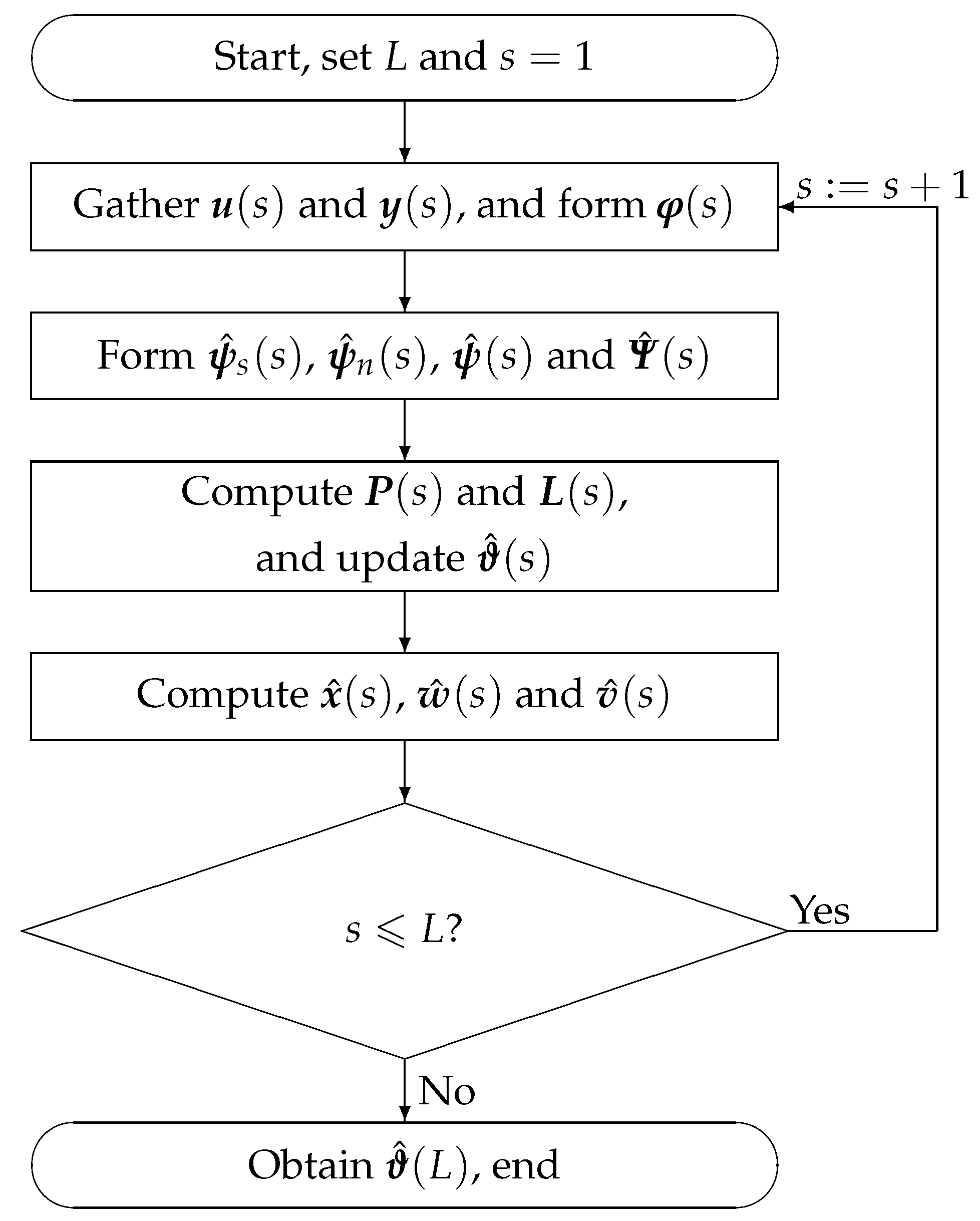
Mathematics Free Full Text Recursive Algorithms For Multivariable Output Error Like Arma Systems Html
A It is used to.

. G Since Aluminum has the largest negative reduction potential we know that it will be the reducing agent Als is being oxidized. Which graph represents an endothermic reaction. Located the farthest from the ribose sugar it has a higher energy than either the α- or β-phosphate.
2N2O g2N2 gO2 g 3NO2 gH2O l2HNO3 lNO g 6CO2 g6H2O gC6H12O6 s6O2 g 3O2 g2O3 g. D The value of ΔG does not depend on the temperature of the reaction. If we were calculating cell potential we would flip this half-reaction to show oxidation potential.
However this reaction takes many years to proceed in the absence of enzyme. Which of the following would most likely be TRUE. Why is this the case.
Which change is exothermic. This problem has been solved. The reaction would result in products with a greater free energy content than in the initial reactants.
_____ When chemical reaction that has a negative Delta G is correctly described as A Endergonic reaction. The reaction would result in an increase in entropy S and a decrease in the energy content H of the system. 2 H₂Sg 3 O₂g 2 H₂Og 2 SO₂g A S has a large negative value.
2H2Og 2H2g O2g Which of the following statements must be true. This also means that endergonic reaction cannot occur spontaneously like exergonic reactions. The reaction is exothermic.
The catalyzed reaction will have the same G. C S has a large positive value. The entropy will usually increase when.
A reaction has a G of -56 kcalmol. B S has a small negative value. 2 It is eothermtc and has a positive AH.
A chemical reaction that has a positive ΔG is best described as _____. The equilibrium lies far to the right. The reaction is slow.
2 It is exothermic and has a positive H. D When ΔH for a reaction is negative the reaction is never spontaneous. E When ΔH for a reaction is very positive the reaction is not expected to be spontaneous.
A -- B C heat. Which reaction is most likely to have a positive ΔSsys. Endergonic exergonic exothermic and endothermic.
That is when H2Og is introduced into a flask no O2 or H2 will form even over a long period of time. Which of the following best describes the reaction. It can also be described as an endergonic reaction - that is a chemical reaction in which the standard change in free energy is positive and energy is absorbed.
A reaction occurs that results in an increase in the number of moles of gas. Biology is brought to you with support from the Amgen Foundation. An example of such a reaction is conversion if carbon dioxide and water into glucose in photosynthesis.
Which reaction is most likely to have a positive. C When ΔG for a reaction is zero the system is at equilibrium. Which statement best describes a chemical reaction in which energy is released.
Which of the following reactions have a positive delta S rxn. E S is zero. B When ΔG for a reaction is positive the reaction is nonspontaneous.
A man pulls a box with a force of 70N along the positive y-axiswhile a boy pulls it with a force of 50Nmaking. This is the currently selected item. 4 It is endothermic and has a positive AH.
This reaction does not obey the second law of thermodynamics. _____ When chemical transport or mechanical work is done by an organism what happens to the heat generated. The endergonic reaction takes free every from the environment.
The following reaction has a positive value of ΔG. 4 It is endothermic and has a positive H. E none of these statements are true.
A particular reaction has a positive delta G. Endergonic exergonic exothermic and endothermic. 7 Given the reaction H g 1202g H20l 286 kJ.
A solid changes to a liquid. The initial free energy of the reactants is much less than the final free energy of the products. If you double the amount of enzyme in the reaction what will be the G for the new reaction.
ATP and reaction coupling. The catalyzed reaction will have higher activation energy. During a laboratory experiment you discover that an enzyme-catalyzed reaction has a G of -20 kcalmol.
A molecule is broken into two or more smaller molecules. 6 Which statement best describes a chemical reac- tion in which energy is released. 1 It is exothermic and has a negative H.
Gibbs free energy and spontaneous reactions. Check all that apply 1 As2Bg --Cg 2 2Ag3Bg --4Cg 3 2Ag2Bg --5Cg 4 2AgBs --3Cg Not sure how to tell if the reaction is positive. Question 7 1 1 pts Which of the following aspects of enzyme structure is best described by a clasping handshake analogy.
1 It is exothermic and has a negadve AH. 3 It is endothermtc and has a negaWe AH. The reaction will not occur.
C ΔG is positive for reactions that have to much reactant in the standard state. D S has a small positive value. 1 3 2 4 6.
This therefore means the free energy of products is higher than that of reactants.

Recruitment Sources Presentation Graphics Templates Powerpoint Presentation S Powerpoint Presentation Slides Presentation Slides Templates Placement Agencies

Kopioppi Quotes Quotesindonesia Katakata Katacewek Katagalau Katahati Patahhati Cinta Bicarahati Isihati Patah Hati Kata Kata Indah Kutipan Remaja
No comments for "Reaction That Has a Positive Δg Is Best Described as"
Post a Comment