How Many Unpaired Electrons Does Mn Have
In the complex ion ML6n Mn has six d electrons and L is a weak field ligand. What is unpaired electrons in ground state.

Number Of Unpaired Electrons Of Neutral Manganese Atoma And Its Divalent Youtube
Here the electron configuration of nitrogen shows that three unpaired electrons exist.

. How many unpaired electrons does MN 4 have. How many unpaired electrons does nitrogen have. Аdditionally how many unpaired electrons does mn 3 have.
What is the electron configuration of mn2. Its complete electron configuration is 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁵. Four unpaired electrons.
Number of unpaired electrons in mn3 is. Magnesium has only two valence electrons. Thus Mn4 has three unpaired electrons.
How many unpaired electrons are there in MN 3. 5291 College Avenue in Oakland CA 94618. Asked Jun 26 2017 in Chemistry by Lola_M.
How many unpaired electrons does MN 4 have. Answer Number of 3d electrons. The electronic configuration of Mn3 is Ar3d44s0.
SOLVEDHow many unpaired electrons are in the fol Learn this topic by watching the electron configuration concept videos. The ligands are weak field ligands. Why chlorine acts as a strong field ligand in tetrachloridoplatinateII.
Since Manganese has atomic number 25 it means it has 25 electrons. Ar 4s0 3d5It looks like that 5 or 3 or at least 1 one electrons are. What is the coordination number for Co in Mg Co enBr42.
How many unpaired electrons does Mn have in Mn CN63- ion. Is the ion paramagnetic or diamagnetic. Manganese has atomic number 25.
Therefore this octahedral complex has more unpaired electrons than the tetrahedral complex. The manganese has 5 unpaired electrons. Number of unpaired electrons in MnCN_63- is What is the number of unpaired d-electrons which the element Mn can have in the compound.
Does boron have 3 unpaired electrons. Alden cordovan shoes for sale. Mn is configured.
So there are 4 unpaired electrons in 3d. There are four unpaired electrons. In the complex ion ml6n mn has six d electrons and l is a weak field ligand.
Therefore it would need to pair up with other atoms in the periodic table to fulfil the octet rule As for the number of unpaired electrons there should be 2 unpaired electrons for Mg when it is on its own. A Mn atom has 5 electrons in its 3d subshell. A 1 b 2 c 4 d 5 e 0.
2 unpaired electrons. How many valence electrons does manganese ionmn 2mn 3mn 4 have. Cashmere short sleeve football manager 2022 4-4-2 how many unpaired electrons does nitrogen have.
How many unpaired electrons does fe2 have. 5 Explanat View the full answer. Mn has oxidation number 3 so Mn3 has electrong configuration Ar3d4.
How many unpaired electrons are in iron. 5 Number of unpaired electrons. The manganese atom exhibits mn 2 mn 3.
Since Mn3 losses 3 electrons then Mn becomes 3d4 hence it has 4 unpaired electrons. How many ions does it dissociate into when dissolved in water. Ar 4s2 3d5 soMn2 has an electron configuration of.
Cr have 6 unpaired electrons but Mn has 5 unpaired electrons. How many unpaired electrons will there be in a low spin octahedral complex of Fe II. Is Cl A strong or weak ligand.
The hexaquo manganeseII ion contains five unpaired electrons while the hexacynoion contains only one unpaired electron. All those 5 electrons are unpaired. Which of the following is true for Co OH63-.
One 4s electron can be excited to the 4p orbital and this gives the atom 7 unpaired electrons and hence Mn exhibits more oxidation states than Cr.

How Many Unpaired Electrons Does Manganese Have Lisbdnet Com

How Many Unpaired Electrons Does Manganese Have Lisbdnet Com

The No Of Unpaired Electrons In Mn 7 Ions At No Of Mn 25 Is Youtube

How Many Unpaired Electrons Are In An Atom Of Manganese Study Com
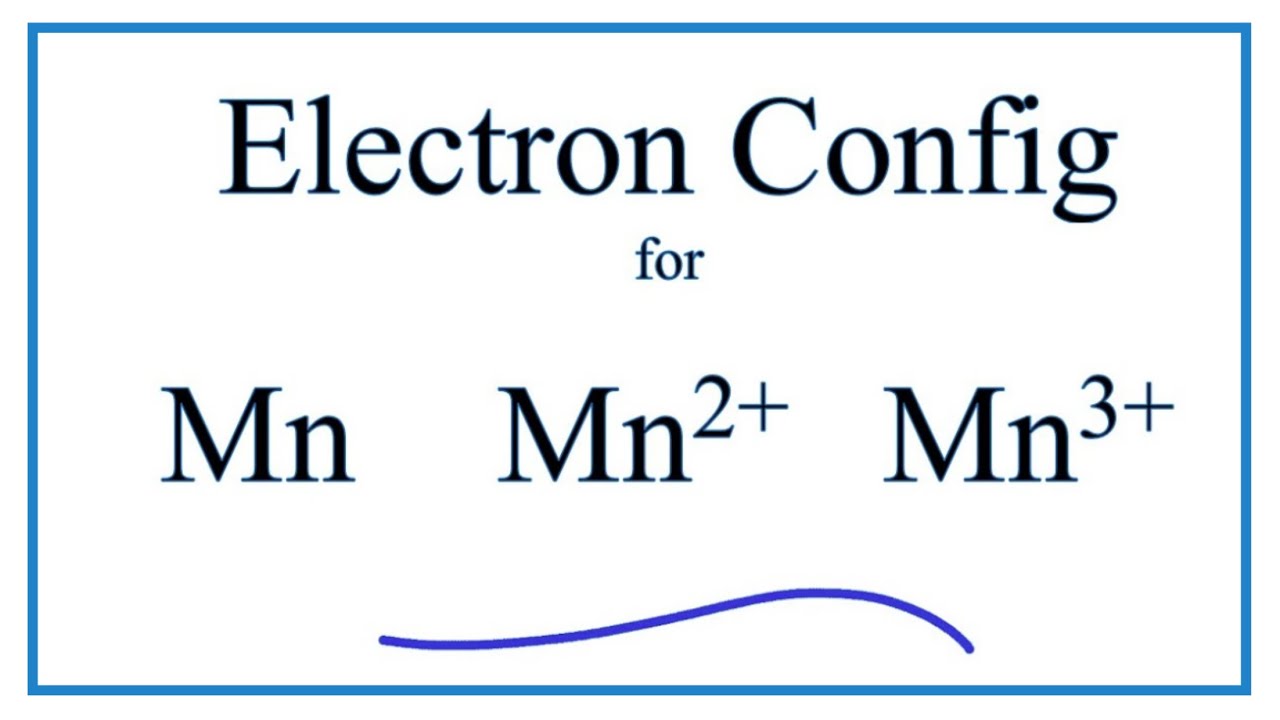
How Many Unpaired Electrons Does Mn Have Lisbdnet Com

How Many Unpaired Electrons Does Mn Have Lisbdnet Com

The Number Of Unpaired Electron In Mn 4 Z 25 Is Youtube

No comments for "How Many Unpaired Electrons Does Mn Have"
Post a Comment